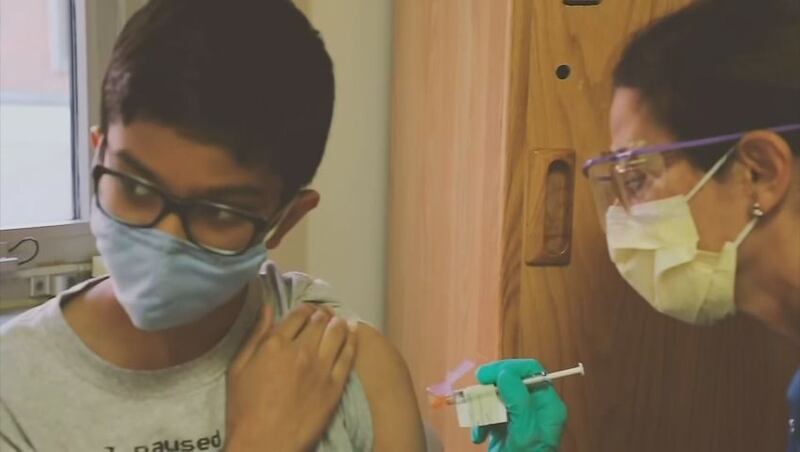
Pfizer is recruiting and already testing their COVID-19 vaccine in kids 12 years and older. It’s their latest effort to get the vaccine ready for children before school begins next year.
Moderna has also started the process to get their clinical trial for kids underway.
The Benaroya Research Institute at Virginia Mason in Seattle is the only location in Western Washington currently listed on clinicaltrials.gov as part of Pfizer’s expanded vaccine clinical trial that includes children.
Virginia Mason said on Friday it has not recruited any kids as part of the trial so far and didn’t have information on when that could change.
While most parents are eager for the day a COVID-19 vaccine is available and approved for kids, some are also concerned about enrolling their kids in a vaccine trial.
“Probably not a trial. But thank you to all the pioneers who are doing that,” said Rayne Capps, a Seattle parent. “Just having your own kids be the center of research, I’m a little skeptical of it,” she said.
“With being youth and not really being able to make their own decisions —doesn’t really seem something that I would be into doing,” said Kyle Capps, also a Seattle parent.
Pfizer and Moderna are both starting the trial with testing in older kids first. Moderna has said it plans to start testing on children younger than 11 years old sometime in 2021.
The goal is to have a vaccine ready for kids before the school year next fall.
“I’m so happy to hear that recruitment is beginning to bring children into these trials,” said Dr. Danielle Zerr, chief of infectious diseases at Seattle Children’s Hospital.
She says the difference in how COVID-19 affects children shows their immune systems are different from adults’, and the vaccine may work a bit differently in kids. For example children may need a smaller dose.
But she says any reaction to the vaccine will likely be similar to what we’re seeing in adults.
“That’s why we do the studies, is to learn that,” Zerr said. “With the adult data, the most common side effects are the usual side effects we see with vaccines like soreness at the site and fever,” she said.
Some parents said they would sign up their kids.
“If she feels comfortable enough – yes. I’d want that to be her decision, I don’t ever want to force her to do something like that, you know? If she feels like she’s up for the challenge, then yes,” said Ruth Garcia, another Seattle parent.
Dr. Zerr says it’s a good idea for parents to start thinking about the COVID vaccine for their kids now.
“The COVID vaccine is top of many people’s minds, and I think it’s a great idea to start talking about it now and start planning for it,” Zerr said. “I think it should be a society priority really to get kids back to school in the fall,” she said.
The United Kingdom has approved the Pfizer vaccine for use in adults, and teens as young as 16 years old.
Both the Moderna and Pfizer vaccines use mRNA technology – not the actual virus – which means you cannot get COVID-19 from taking the vaccine or participating in a clinical trial.
Cox Media Group